In this Article
- Defining the Engine of R&D: Recombinant Protein/DNA Technology
- Precision Engineering: Mastering Recombinant Protein Production
- Recombinant Proteins: Enabling Breakthrough Applications in R&D
- The Horizon: Future Trends in Recombinant Protein Technology
- Conclusion: Recombinant Proteins as Essential Tools for Scientific Advancement
Advancing Scientific Frontiers Through Recombinant Protein Technology
All of the products listed in AAA Biotech’s catalog are strictly for research-use only (RUO).
Key Takeaways/Learnings
- Recombinant proteins require native folding and bioactivity for reproducible research.
- Host cell selection determines protein complexity, yield, and modifications.
- Stringent QC ensures lot-to-lot consistency for reproducibility.
- 3D organoids rely on high-quality growth factors for differentiation.
- AI/ML and CRISPR accelerate production efficiency and reduce costs.
- Specialized services eliminate validation cycles and accelerate discoveries.
Modern biological and biomedical research thrives on precision. Gone are the days when researchers could rely solely on crude biological extracts to drive high-stakes experiments.
Today, the cornerstone of reproducible and cutting-edge discovery is access to molecular tools that are highly defined, pure, and functionally validated. This foundation is built upon the sophisticated engineering and utilization of recombinant proteins.
A recombinant protein is, fundamentally, an engineered molecular tool. It is a protein whose specific genetic sequence has been cloned into an expression vector and introduced into a host cell system, such as mammalian cells, bacteria, or yeast, to facilitate its expression.1
The crucial distinction that sets some recombinant proteins apart from others for research use, is the recombinant protein’s bioactivity: the molecule must not only be chemically pure, but it must correctly fold and maintain the ability to elicit a specific effect or response within a biological system.1
The complexity of contemporary R&D - particularly in areas like 3D cell modeling and regenerative medicine requires molecular inputs that are precisely controlled. This demand for higher functional fidelity drives the technical complexity of recombinant protein technology.
If a research question involves modeling complex human development, the proteins used (e.g., growth factors) must exhibit native folding and post-translational modifications (PTMs) that are often challenging to replicate. This pursuit of precision is what makes the mastery of recombinant protein production a foundational component of biological scientific advancement.
Defining the Engine of R&D: Recombinant Protein/DNA Technology
RDT forms the very core of modern research.
From Gene to Function: What is a Recombinant Protein?
At its core, a recombinant protein is a molecule produced by combining genetic material from different sources - a process that uses recombinant DNA technology. This allows scientists to produce large, consistent quantities of proteins that are naturally rare or difficult to isolate from their native sources.
For researchers, the value lies in the consistency and high purity of the final product. Reliable scientific outcomes depend on using reagents where every batch maintains documented purity and consistent biological activity.1 This is why research-grade proteins undergo such rigorous quality control specifications.2
Understanding Recombinant Protein Technology
What is recombinant protein technology? It is the comprehensive discipline covering every step from initial gene selection to final product characterization. It is an end-to-end workflow designed to engineer and produce functional molecules.
The general workflow for recombinant protein production consists of four key stages 3:
- Cloning/Construct Design: Construction of expression vectors carrying the target gene of interest.
- Expression: Delivering the construct to the chosen host cells, growing those cells, and inducing the protein expression.
- Purification: Strategic isolation and separation of the target protein from the host cell mixture.
- Analytics/Characterization: Identification, quantitation, and rigorous testing of the isolated protein's purity and function.
The overall objective of the technology is not just protein synthesis, but the reliable production of high-quality, bioactive reagents suitable for demanding applications, including advanced structural studies, protein engineering, and high-throughput screening.3
Precision Engineering: Mastering Recombinant Protein Production
The process of generating research-grade recombinant proteins is a multifaceted and demanding expertise, requiring comprehensive understanding of several areas, including genetic engineering, cell culture, and biochemistry. This is why many leading research groups rely on a dedicated recombinant protein production service to provide validated, ready-to-use materials.
Engineering the Code: The Foundation of Production
The success of recombinant protein production begins long before cell culture commences. It starts with the meticulous design of the expression vector.3
- Construct Design and Optimization: The DNA sequence coding for the protein is obtained, often via gene synthesis, which allows for crucial refinements.
- Codon Optimization: A common refinement step involves codon optimization, overcoming limitations associated with interspecific differences in codon usage. This is essential for maximizing protein production yield in the chosen host system.4
- Incorporation of Tags: Sequences coding for tags (e.g., His-tags) are integrated into the construct. These tags facilitate efficient protein purification and detection, and sometimes improve the overall expression level and solubility of the target protein.4
The Expression Phase: Types of Recombinant Proteins and Host Selection
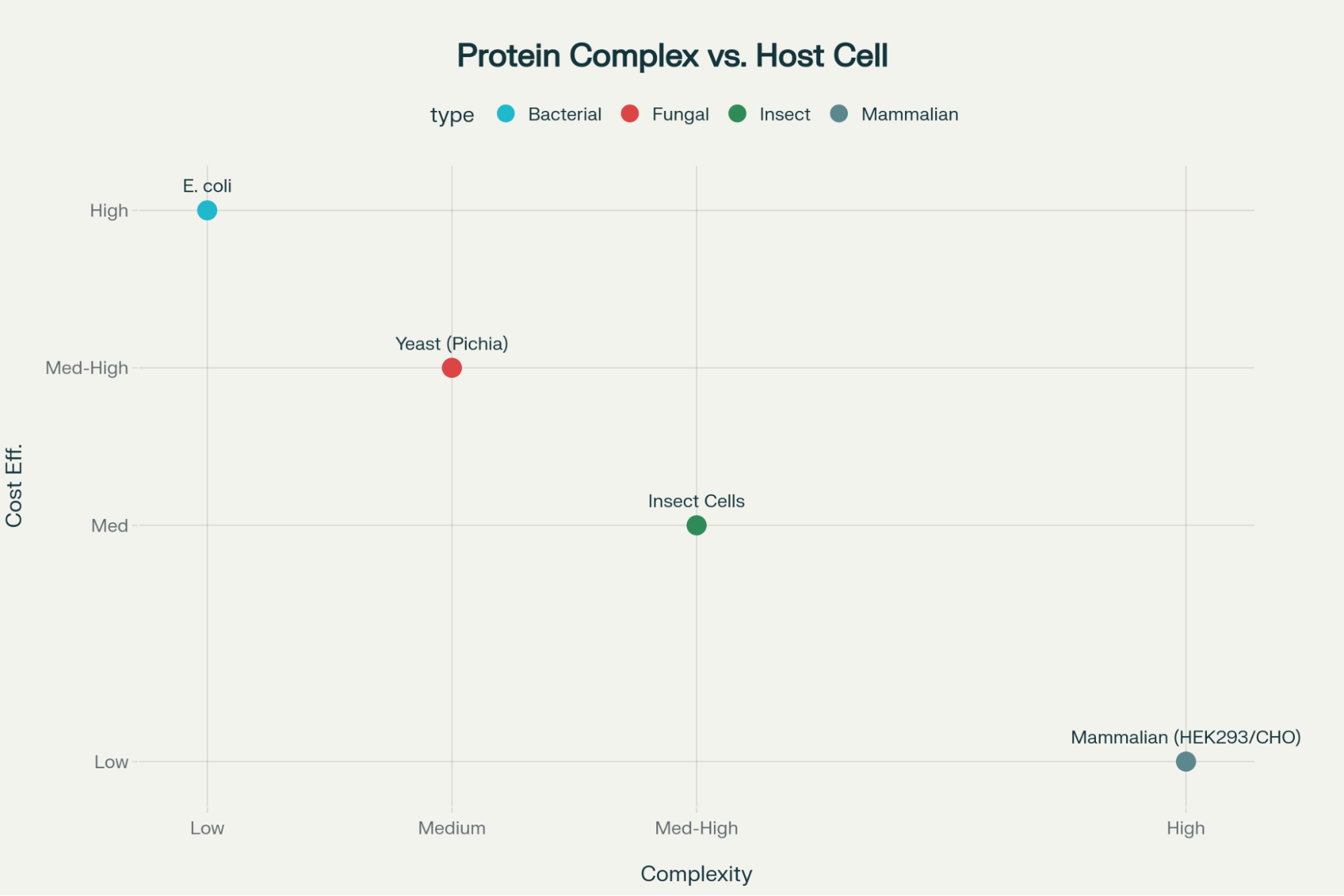
Choosing the appropriate host cell system is perhaps the most critical decision in recombinant protein production, as it dictates the complexity and functional fidelity of the resulting protein. The choice balances factors like cost, speed, final yield, and the need for specific post-translational modifications (PTMs).5
The different types of recombinant proteins produced often correlate directly with the expression system utilized:
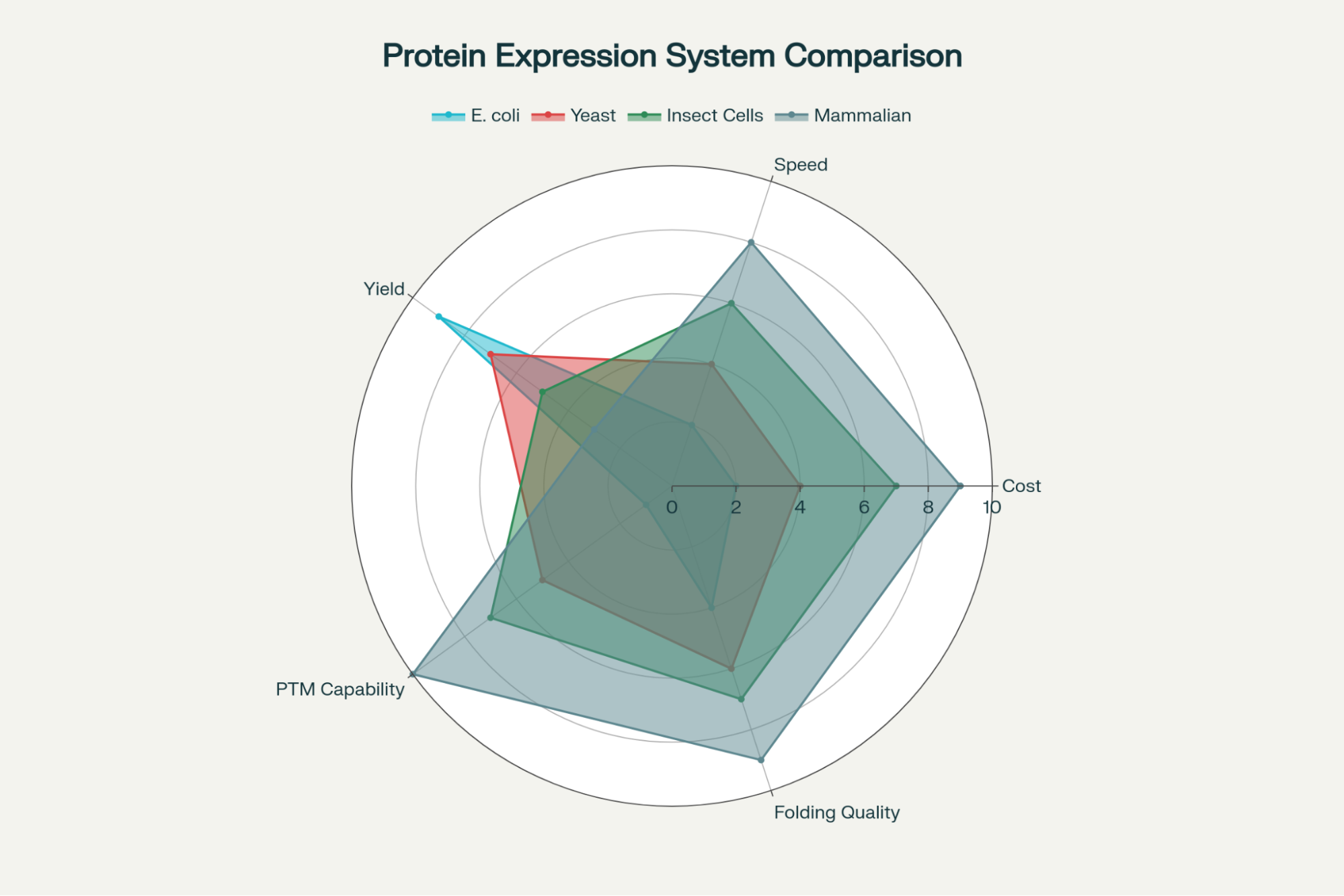
- Bacterial Systems (e.g., E. coli): These are favored for high yield, fast growth, and low cost. They are ideal for simple proteins that do not require complex modifications. However, a significant technical hurdle is that complex proteins expressed in bacteria often misfold and aggregate into insoluble inclusion bodies.4
- Mammalian Systems (e.g., CHO, HEK293): Although these systems incur higher costs and typically result in lower yields, they are indispensable for producing proteins (like bioactive growth factors and complex cytokines) that require native mammalian folding and PTMs, such as glycosylation.5 These functional aspects are essential for research involving human cell culture and complex signaling pathways.
- Yeast and Insect Cells: These systems offer an intermediate solution, providing some capability for PTMs while often delivering higher yields than mammalian cells.5
The trade-off between speed/cost (bacterial systems) and native function (mammalian systems) highlights a major technical challenge for researchers. If a complex cytokine is produced in E. coli, the gain in yield is often offset by the considerable risk and labor required for subsequent denaturation, refolding, and validation to ensure biological function.4 This core difficulty underscores the value delivered by a specialized recombinant protein production service.
| S.No | Expression System | Advantages (Typical Uses) | Disadvantages (Considerations) |
|---|---|---|---|
| 01. | E. coli (Bacterial) | High yield, low cost, fast growth. Ideal for simple, non-glycosylated proteins. | Lack of PTMs often requires refolding from inclusion bodies (misfolding). |
| 02. | Yeast (Pichia pastoris) | Higher yields than mammalian cells; capable of basic glycosylation; relatively fast. | PTMs may differ significantly from native mammalian proteins. |
| 03. | Insect Cells (Baculovirus) | High-level expression; capable of complex PTMs (closer to mammalian); generally high solubility. | Requires specialized cell culture expertise; higher complexity and cost than bacteria/yeast. |
| 04. | Mammalian Cells (HEK293, CHO) | Best native folding and glycosylation/PTMs. Essential for complex human bioactive proteins (cytokines). | Low yield; high cost; complex, time-consuming culture maintenance. |
Table 1: Key Characteristics and Selection Criteria for Recombinant Protein Expression Systems
Downstream Processing: Achieving Research-Grade Purity
Obtaining a protein that is pure enough for functional research requires a carefully planned series of downstream processing steps.
- Extraction and Stabilization: Cells are lysed to release the protein, and specific inhibitors are added to prevent the degradation of the target molecule.5 If the protein is in inclusion bodies, extraction requires harsh denaturing buffers, followed by crucial refolding to restore native function.4
- Capture and Polishing: Initial isolation often uses affinity chromatography (leveraging engineered tags). Subsequent steps, such as ion exchange chromatography and size-exclusion chromatography (SEC), are essential for enhancing purity.5 This stage of documented purity is critical 6 for any downstream research endeavor seeking high fidelity and low background noise. Researchers can explore advanced purification techniques to ensure the highest quality materials for functional studies.
- Quality Control (QC): Research success is critically dependent on the integrity and functional quality of the proteins used.1 Stringent QC involves 7:
- Identity and Purity Verification: Techniques like SDS-PAGE, mass spectrometry, and immunological assessments, such as Western blotting, are used to ensure the isolated protein is the correct molecule and free from contaminants.3
Learn more about verifying purity using Western blotting techniques: Western Blot Guide: Steps, Uses & Key Role in Research - Bioactivity Testing: The protein's function must be confirmed using appropriate assays, such as specialized cellular bioassays or quantitative systems like ELISA.7 AAA Biotech maintains industry-leading bioactivity and strict lot-to-lot consistency specifications.2 High-quality protein standards are vital for optimizing analytical protocols.
- Specialized Grades: For highly sensitive applications, proteins are often offered in Animal-free grades (manufactured using only chemically defined, animal-origin free materials) or GMP-grade, which is necessary when research protocols are being prepared for potential technology transfer.7 Using these defined materials from the start minimizes the lengthy re-validation process required to transition discoveries into controlled environments.
The requirement for exceptional purity and functional validation in the R&D production phase serves a dual purpose: it guarantees the reliability of current experiments, and it inherently establishes the standardized, high-quality antigens necessary for the initial stages of development of diagnostic tests and complex research assays.
Recombinant Proteins: Enabling Breakthrough Applications in R&D
The application of recombinant protein extends across the most ambitious frontiers of life science, providing the necessary molecular inputs to transition from theoretical concepts to reproducible experimental systems.
01. Guiding Cell Fate in Regenerative Medicine and Tissue Engineering
Recombinant proteins, particularly cytokines and growth factors, are central to steering the complex process of cellular differentiation. They act as essential signaling agents that direct stem cells to differentiate into specific tissue types, a process that is fundamental to regenerative medicine and tissue engineering.10
- Synthetic Gamete Development: In the global quest to conserve critically endangered species, researchers rely on these proteins to transform somatic cells (like skin cells) into induced pluripotent stem cells (iPSCs).11 Recombinant proteins are crucial for maintaining the iPSCs’ pluripotency and then directing their differentiation into artificial gametes.11 This approach has been successfully applied to species like the Giant Panda and the functionally extinct Northern White Rhinoceros.
- Advanced Organoid Modeling: Recombinant growth factors are pivotal for culturing complex 3D organoids, which far surpass 2D systems in mimicking in vivo organ structure and function.9
- For example, scientists created a human skin model entirely from pluripotent stem cells by orchestrating differentiation using specific recombinant proteins in the culture media. This resulted in complex skin organoids complete with functional hair follicles and nerves. 11
- Even research conducted in extreme environments, such as culturing iPSCs aboard the International Space Station, depended on recombinant growth factors in the media to maintain cell viability and pluripotency under microgravity conditions. 11
02. Accelerating Viral and Disease Research Models
High-purity recombinant proteins are foundational reagents in the study of pathogens and complex physiological systems, allowing for precise characterization and modeling of disease dynamics.
- Recombinant Viral Proteins for Pathogen Study: Purified recombinant viral proteins or other pathogen antigens are fundamental for investigating viral replication dynamics and characterizing host-pathogen interactions in vitro. 11
- Next-Generation Disease Modeling: Recombinant growth factors were used to differentiate and maintain 3D bat lung organoids, creating models that accurately emulate organ structure and tissue function relevant to bat-derived infectious diseases like SARS-CoV-2. 11 This model captures key hallmarks of bat respiratory biology and antiviral defenses, a significant advantage over oversimplified 2D cell lines.
- Unraveling Fundamental Biology: In basic biology research, recombinant proteins enabled the creation of specialized intestinal organoids. These 3D in vitro cell models were instrumental in studying the gut-brain axis, allowing researchers to precisely track and manipulate human gut receptors to understand how specialized sensor cells distinguish real sugar from artificial sweeteners. 11
The reliance on recombinant proteins for complex 3D organoid research signifies a paradigm shift: R&D now relies on testing compounds and theories on dynamic, functional systems that require active, molecular guidance. The quality of the recombinant protein directly determines the validity and complexity of the model itself.
| Research Domain | Recombinant Protein Class | Specific R&D Use Case |
|---|---|---|
| Regenerative Medicine | Growth Factors, Cytokines (e.g., IL-7, PDGF-BB) | Inducing differentiation of iPSCs into specific lineages, supporting the manufacture of cell therapy drugs, and stem cell expansion 11, 9. |
| Organoid Development | Defined Growth Factors and Signaling Molecules | Culturing complex 3D models, such as human skin organoids with functional hair follicles and bat lung organoids 11. |
| Pathogen Research | Recombinant Viral and Pathogen Proteins | Characterizing host antiviral defenses and investigating viral replication dynamics in specialized in vitro models 11. |
| Assay Standardization | High-Purity Antigens and Standards | Establishing robust protocols for large-scale research screening and high-throughput assays (e.g., ELISA) 12. |
Table 2: Recombinant Protein Use in Advanced Fundamental Research Models
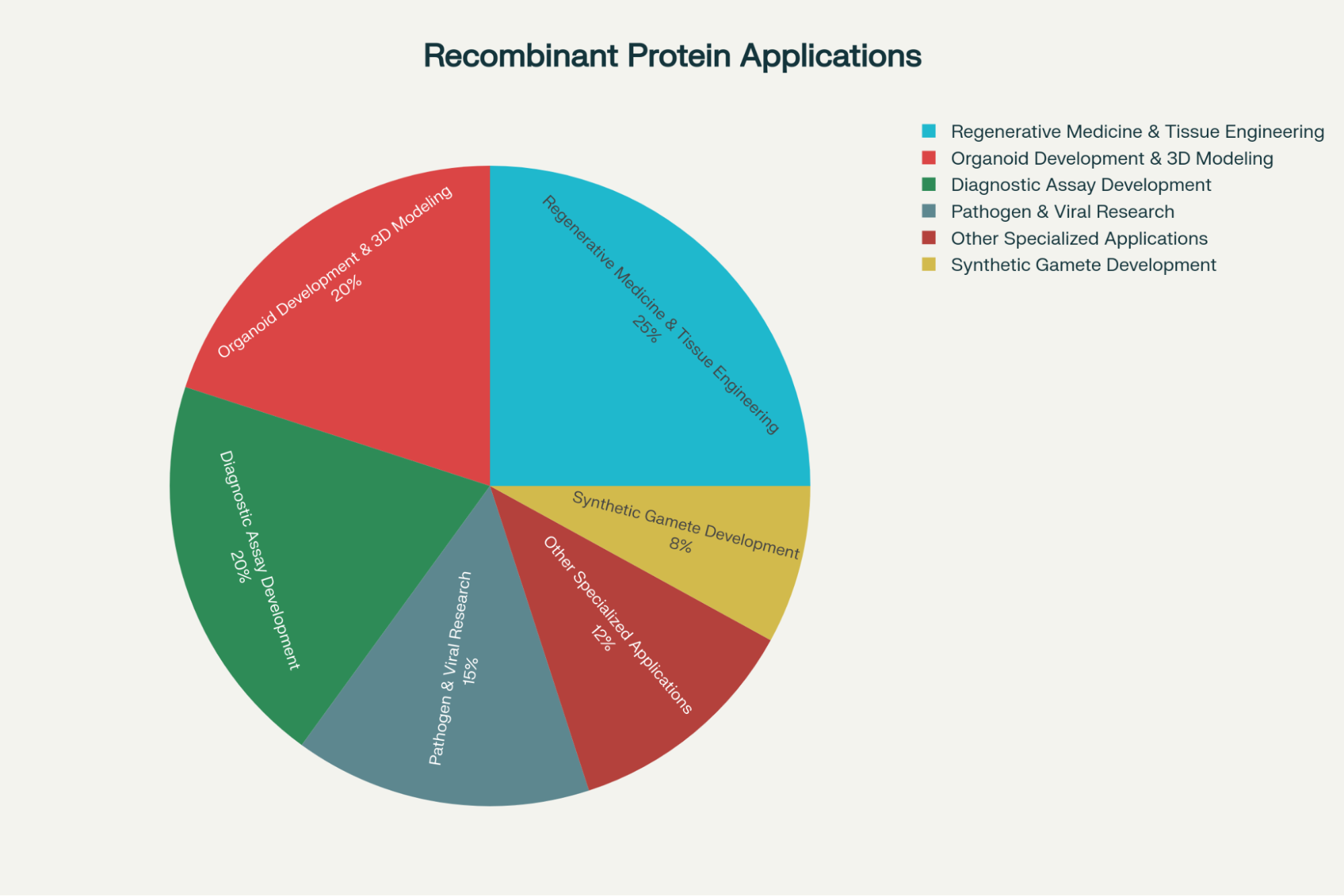
03. Standards for Research Assay Development
The production of pure, consistent recombinant viral proteins or antigens is essential for establishing standardized research protocols. For example, fundamental research into infectious diseases, such as studies focusing on Mycoplasma bovis, has shown that recombinant proteins are predominantly used in the development of highly effective, indirect enzyme-linked immunosorbent assays (ELISA) for research use.12
By focusing on the documented purity and batch-to-batch consistency of recombinant proteins, researchers are able to establish reliable standards for high-throughput screening and detailed analytical studies. This rigorous R&D work, essential for the initial stages of the development of diagnostic tests research, ensures that the resulting protocols are accurate and reproducible, achieving high research sensitivity and specificity
See: Mitigating Cross-Reactivity In Antibody Testing: Precise Strategies For Reliable Results
The ability to translate successful antigen usage into standardized field applications relies entirely on the quality achieved during the recombinant protein production phase.12
The Horizon: Future Trends in Recombinant Protein Technology
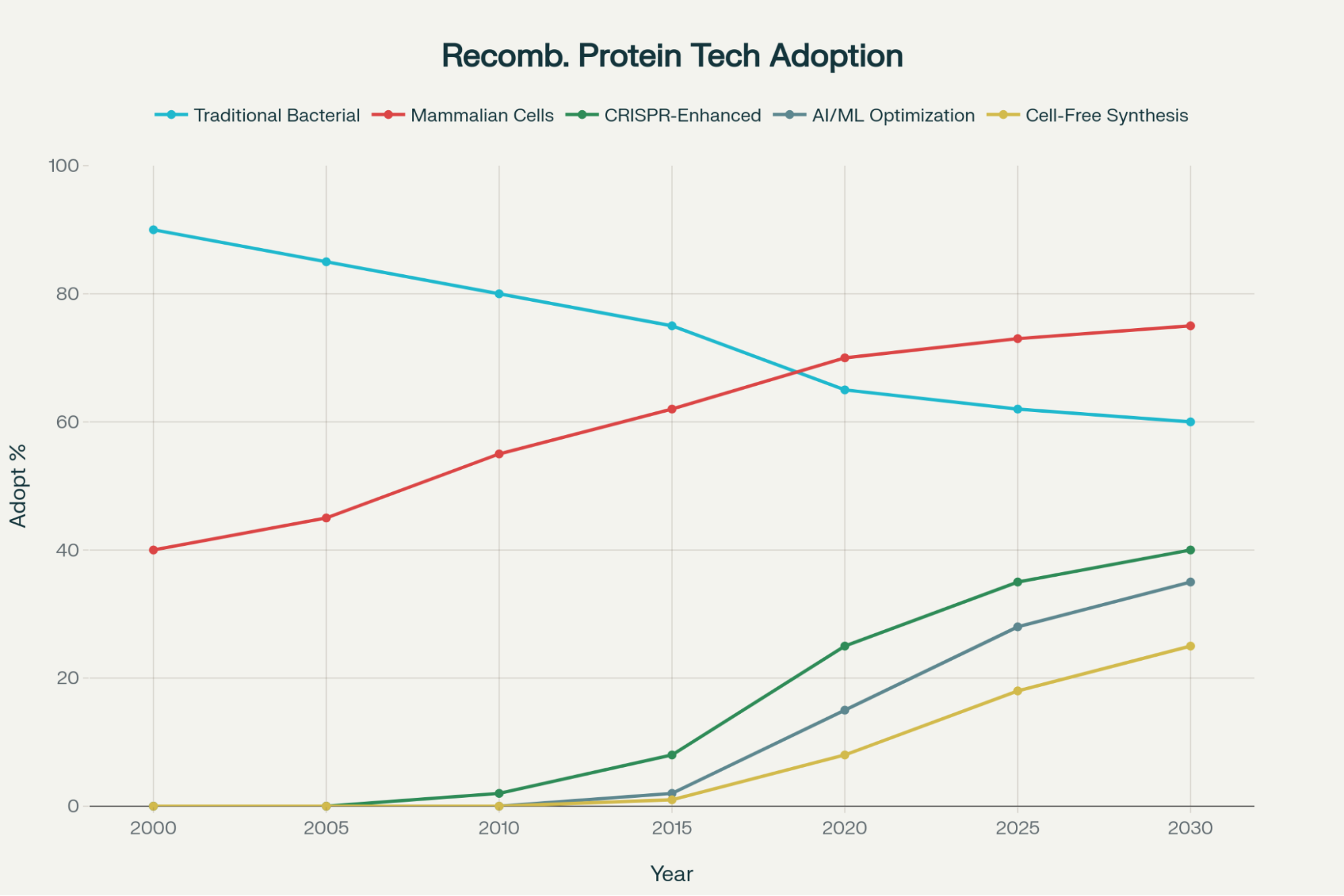
The future of recombinant protein technology is being shaped by cutting-edge advancements that promise enhanced efficiency, precision, and sustainability, rapidly evolving the capabilities of the recombinant protein production service industry.
01. AI and Machine Learning: Optimizing Production
The most exciting prospective development in recombinant protein technology is the integration of artificial intelligence (AI) and machine learning (ML).13
- Accelerating Optimization: AI algorithms are designed to analyze vast biological datasets to predict optimal protein structures, solubility, and stability. This reduces the need for expensive, time-consuming empirical screening, which has historically been a major bottleneck in protein development.
- Data-Driven Design: This data-driven approach predicts the best expression conditions and identifies potential production issues early in the R&D process, significantly accelerating the pipeline for producing novel or specialized proteins.13 By streamlining production and reducing costs, AI directly facilitates the high-volume production of specific antigens necessary for using recombinant proteins for diagnosis in high-throughput research assays.
02. Advanced Genetic Engineering and Novel Systems
Technological diversification is enabling greater control over the host systems used for production.
- CRISPR-Enhanced Hosts: Advances in genetic engineering, particularly the use of CRISPR/Cas9, are allowing researchers to make precise modifications to the host cell genome.13 This ability to fine-tune the cellular machinery enhances protein expression and functionality, maximizing production yield, particularly for complex and sensitive types of recombinant proteins.13
- Evolving Expression Platforms: The field is diversifying beyond traditional expression hosts to meet highly specialized structural and scaling requirements:
- Cell-free Synthesis: This system offers unparalleled speed and granular control over the environment, bypassing many cellular constraints.
- Plant-Based Systems: These offer scalable, sustainable alternatives for producing large volumes of certain proteins, providing unique structural possibilities.13
This proliferation of novel expression systems indicates that future R&D will require extreme production specificity. A recombinant protein production service must evolve to offer highly customized, application-specific host portfolios, thereby providing the exact molecular tool needed for each unique research challenge.
03. Sustainability in Bioprocessing
Sustainability is becoming an increasingly important consideration in the recombinant protein production process.13 Researchers are exploring eco-friendly methods aimed at reducing the environmental footprint of large-scale bioprocessing. This includes prioritizing the use of renewable raw materials, energy-efficient processes, and strategic waste reduction throughout the purification workflow.
Conclusion: Recombinant Proteins as Essential Tools for Scientific Advancement
Recombinant proteins are the essential molecular tools that power the most ambitious research projects in modern biological science. From the precise engineering of complex 3D organoids capable of growing functional hair follicles to the development of robust protocols for regenerative medicine, success depends entirely on the purity, consistency, and validated bioactivity achieved during recombinant protein production.1
The complexity of contemporary R&D validates the need for rigorous, specialized production services. By choosing high-quality, research-grade proteins, scientists can ensure their results are reproducible and their discoveries are poised for future technological translation.
The integration of forward-looking methodologies, such as AI optimization and CRISPR-enhanced systems, ensures that recombinant protein technology will continue to evolve, overcoming current limitations in complexity, cost, and speed, and making tomorrow's breakthroughs possible today.13
We invite you to explore our extensive catalog of research-grade recombinant proteins, developed under rigorous quality control standards, or contact us for a personalized consultation on custom recombinant protein development tailored to your unique research needs.
Contact Us and Request a Quote.
Faq's
1. What are the key criteria for selecting an optimal expression system for R&D?
The choice depends on the protein’s complexity and intended use. Simple proteins can use bacteria for high cost-effectiveness and yield, but complex mammalian proteins requiring native folding and post-translational modifications (PTMs) must be produced in mammalian hosts like HEK293 or CHO cells.
2. How does stringent quality control (QC) impact research reproducibility?
Stringent QC, including tests for purity, identity (Western Blot), and biological activity (ELISA), ensures high lot-to-lot consistency. This consistency is critical because variations in key reagents can invalidate complex downstream experiments, such as advanced organoid differentiation protocols.
3. What role do recombinant proteins play in 3D organoid research?
They act as essential molecular architects, specifically as growth factors and cytokines, guiding complex cellular differentiation. They move cells through embryonic stages to form functional, multi-layered models like skin or lung organoids, overcoming the structural limitations of 2D culture.
4. How is recombinant protein production evolving through technology?
Future production leverages AI/ML to optimize protein design and expression conditions, significantly accelerating development. Additionally, advanced genetic engineering, such as CRISPR/Cas9, is used to refine host cells for improved yield and maximum functional fidelity.
5. Why is protein refolding necessary after bacterial expression?
Bacterial systems often express complex proteins as insoluble aggregates called inclusion bodies. Refolding, using progressive buffer exchange after extraction with denaturing buffers, is required to restore the protein’s native three-dimensional structure and its essential biological function.








