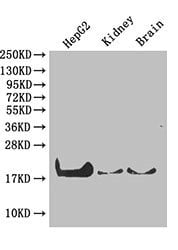
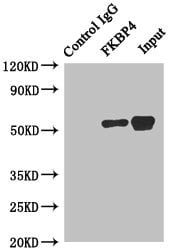
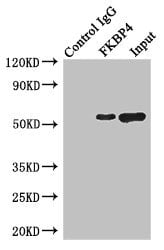
Peptidyl-prolyl cis-trans isomerase A Recombinant Protein | PPIA recombinant protein
Recombinant Human Peptidyl-prolyl cis-trans isomerase A
Gene Names
PPIA; CYPA; CYPH; HEL-S-69p
Purity
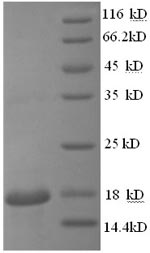
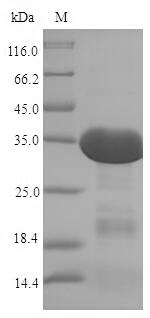
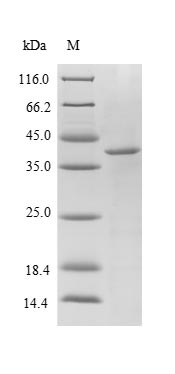
Greater or equal to 85% purity as determined by SDS-PAGE.
Synonyms
Peptidyl-prolyl cis-trans isomerase A; N/A; Recombinant Human Peptidyl-prolyl cis-trans isomerase A; Cyclophilin A; Cyclosporin A-binding protein; Rotamase A; PPIA recombinant protein
Host
E Coli or Yeast or Baculovirus or Mammalian Cell
Purity/Purification
Greater or equal to 85% purity as determined by SDS-PAGE.
Form/Format
Lyophilized or liquid (Format to be determined during the manufacturing process)
Sequence Positions
2-165aa; Full Length of Mature Protein
Sequence
VNPTVFFDIAVDGEPLGRVSFELFADKVPKTAENFRALSTGEKGFGYKGSCFHRIIPGFMCQGGDFTRHNGTGGKSIYGEKFEDENFILKHTGPGILSMANAGPNTNGSQFFICTAKTEWLDGKHVVFGKVKEGMNIVEAMERFGSRNGKTSKKITIADCGQLE
Preparation and Storage
Store at -20 degree C, for extended storage, conserve at -20 degree C or -80 degree C.
Related Product Information for PPIA recombinant protein
PPIases accelerate the folding of proteins. It catalyzes the cis-trans isomerization of proline imidic peptide bonds in oligopeptides.
Product Categories/Family for PPIA recombinant protein
References
Complementary DNA for human T-cell cyclophilin.Haendler B., Hofer-Warbinek R., Hofer E.EMBO J. 6:947-950(1987) Characterization of the human cyclophilin gene and of related processed pseudogenes.Haendler B., Hofer E.Eur. J. Biochem. 190:477-482(1990) Complete sequencing and characterization of 21,243 full-length human cDNAs.Ota T., Suzuki Y., Nishikawa T., Otsuki T., Sugiyama T., Irie R., Wakamatsu A., Hayashi K., Sato H., Nagai K., Kimura K., Makita H., Sekine M., Obayashi M., Nishi T., Shibahara T., Tanaka T., Ishii S., Yamamoto J., Saito K., Kawai Y., Isono Y., Nakamura Y., Nagahari K., Murakami K., Yasuda T., Iwayanagi T., Wagatsuma M., Shiratori A., Sudo H., Hosoiri T., Kaku Y., Kodaira H., Kondo H., Sugawara M., Takahashi M., Kanda K., Yokoi T., Furuya T., Kikkawa E., Omura Y., Abe K., Kamihara K., Katsuta N., Sato K., Tanikawa M., Yamazaki M., Ninomiya K., Ishibashi T., Yamashita H., Murakawa K., Fujimori K., Tanai H., Kimata M., Watanabe M., Hiraoka S., Chiba Y., Ishida S., Ono Y., Takiguchi S., Watanabe S., Yosida M., Hotuta T., Kusano J., Kanehori K., Takahashi-Fujii A., Hara H., Tanase T.-O., Nomura Y., Togiya S., Komai F., Hara R., Takeuchi K., Arita M., Imose N., Musashino K., Yuuki H., Oshima A., Sasaki N., Aotsuka S., Yoshikawa Y., Matsunawa H., Ichihara T., Shiohata N., Sano S., Moriya S., Momiyama H., Satoh N., Takami S., Terashima Y., Suzuki O., Nakagawa S., Senoh A., Mizoguchi H., Goto Y., Shimizu F., Wakebe H., Hishigaki H., Watanabe T., Sugiyama A., Takemoto M., Kawakami B., Yamazaki M., Watanabe K., Kumagai A., Itakura S., Fukuzumi Y., Fujimori Y., Komiyama M., Tashiro H., Tanigami A., Fujiwara T., Ono T., Yamada K., Fujii Y., Ozaki K., Hirao M., Ohmori Y., Kawabata A., Hikiji T., Kobatake N., Inagaki H., Ikema Y., Okamoto S., Okitani R., Kawakami T., Noguchi S., Itoh T., Shigeta K., Senba T., Matsumura K., Nakajima Y., Mizuno T., Morinaga M., Sasaki M., Togashi T., Oyama M., Hata H., Watanabe M., Komatsu T., Mizushima-Sugano J., Satoh T., Shirai Y., Takahashi Y., Nakagawa K., Okumura K., Nagase T., Nomura N., Kikuchi H., Masuho Y., Yamashita R., Nakai K., Yada T., Nakamura Y., Ohara O., Isogai T., Sugano S.Nat. Genet. 36:40-45(2004) Lys-N and trypsin cover complementary parts of the phosphoproteome in a refined SCX-based approach.Gauci S., Helbig A.O., Slijper M., Krijgsveld J., Heck A.J., Mohammed S.Anal. Chem. 81:4493-4501(2009) Lysine acetylation targets protein complexes and co-regulates major cellular functions.Choudhary C., Kumar C., Gnad F., Nielsen M.L., Rehman M., Walther T.C., Olsen J.V., Mann M.Science 325:834-840(2009) Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis.Olsen J.V., Vermeulen M., Santamaria A., Kumar C., Miller M.L., Jensen L.J., Gnad F., Cox J., Jensen T.S., Nigg E.A., Brunak S., Mann M.Sci. Signal. 3:RA3-RA3(2010) Initial characterization of the human central proteome.Burkard T.R., Planyavsky M., Kaupe I., Breitwieser F.P., Buerckstuemmer T., Bennett K.L., Superti-Furga G., Colinge J.BMC Syst. Biol. 5:17-17(2011) Comparative large-scale characterisation of plant vs. mammal proteins reveals similar and idiosyncratic N-alpha acetylation features.Bienvenut W.V., Sumpton D., Martinez A., Lilla S., Espagne C., Meinnel T., Giglione C.Mol. Cell. Proteomics 11:M111.015131-M111.015131(2012) N-terminal acetylome analyses and functional insights of the N-terminal acetyltransferase NatB.Van Damme P., Lasa M., Polevoda B., Gazquez C., Elosegui-Artola A., Kim D.S., De Juan-Pardo E., Demeyer K., Hole K., Larrea E., Timmerman E., Prieto J., Arnesen T., Sherman F., Gevaert K., Aldabe R.Proc. Natl. Acad. Sci. U.S.A. 109:12449-12454(2012) An enzyme assisted RP-RPLC approach for in-depth analysis of human liver phosphoproteome.Bian Y., Song C., Cheng K., Dong M., Wang F., Huang J., Sun D., Wang L., Ye M., Zou H.J. Proteomics 96:253-262(2014) Structure of human cyclophilin and its binding site for cyclosporin A determined by X-ray crystallography and NMR spectroscopy.Kallen J., Spitzfaden C., Zurini M.G.M., Wider G., Widmer H., Wuethrich K., Walkinshaw M.D.Nature 353:276-279(1991) Crystal structure of recombinant human T-cell cyclophilin A at 2.5-A resolution.Ke H., Zydowsky L.D., Liu J., Walsh C.T.Proc. Natl. Acad. Sci. U.S.A. 88:9483-9487(1991) X-ray structure of a decameric cyclophilin-cyclosporin crystal complex.Pfuegl G., Kallen J., Schirmer T., Jansonius J.N., Zurini M.G.M., Walkinshaw M.D.Nature 361:91-94(1993) X-ray structure of a monomeric cyclophilin A-cyclosporin A crystal complex at 2.1-A resolution.Mikol V., Kallen J., Pfluegl G., Walkinshaw M.D.J. Mol. Biol. 234:1119-1130(1993) Crystal structure implies that cyclophilin predominantly catalyzes the trans to cis isomerization.Zhao Y., Ke H.Biochemistry 35:7356-7361(1996) Crystal structure of cyclophilin A complexed with a binding site peptide from the HIV-1 capsid protein.Vajdos F.F., Yoo S., Houseweart M., Sundquist W.I., Hill C.P.Protein Sci. 6:2297-2307(1997) X-ray structures and analysis of 11 cyclosporin derivatives complexed with cyclophilin A.Kallen J., Mikol V., Taylor P., Walkinshaw M.D.J. Mol. Biol. 283:435-449(1998) Crystal structure of calcineurin-cyclophilin-cyclosporin shows common but distinct recognition of immunophilin-drug complexes.Huai Q., Kim H.Y., Liu Y., Zhao Y., Mondragon A., Liu J.O., Ke H.Proc. Natl. Acad. Sci. U.S.A. 99:12037-12042(2002) Crystal structure of human calcineurin complexed with cyclosporin A and human cyclophilin.Jin L., Harrison S.C.Proc. Natl. Acad. Sci. U.S.A. 99:13522-13526(2002) Solution structure of the cyclosporin A/cyclophilin complex by NMR.Theriault Y., Logan T.M., Meadows R., Yu L., Olejniczak E.T., Holzman T.F., Simmer R.L., Fesik S.W.Nature 361:88-91(1993) The NMR solution conformation of unligated human cyclophilin A.Ottiger M., Zerbe O., Guentert P., Wuethrich K.J. Mol. Biol. 272:64-81(1997) Acetylation regulates cyclophilin A catalysis, immunosuppression and HIV isomerization.Lammers M., Neumann H., Chin J.W., James L.C.Nat. Chem. Biol. 6:331-337(2010)
NCBI and Uniprot Product Information
NCBI GI #
NCBI GeneID
NCBI Accession #
NCBI GenBank Nucleotide #
Molecular Weight
17.9 kDa
NCBI Official Full Name
peptidyl-prolyl cis-trans isomerase A isoform 2
NCBI Official Synonym Full Names
peptidylprolyl isomerase A
NCBI Official Symbol
PPIA
NCBI Official Synonym Symbols
CYPA; CYPH; HEL-S-69p
NCBI Protein Information
peptidyl-prolyl cis-trans isomerase A
UniProt Protein Name
Peptidyl-prolyl cis-trans isomerase A
UniProt Gene Name
PPIA
UniProt Synonym Gene Names
CYPA; PPIase A
UniProt Entry Name
PPIA_HUMAN
Similar Products
Product Notes
The PPIA ppia (Catalog #AAA81588) is a Recombinant Protein produced from E Coli or Yeast or Baculovirus or Mammalian Cell and is intended for research purposes only. The product is available for immediate purchase. The immunogen sequence is 2-165aa; Full Length of Mature Protein. The amino acid sequence is listed below: VNPTVFFDIA VDGEPLGRVS FELFADKVPK TAENFRALST GEKGFGYKGS CFHRIIPGFM CQGGDFTRHN GTGGKSIYGE KFEDENFILK HTGPGILSMA NAGPNTNGSQ FFICTAKTEW LDGKHVVFGK VKEGMNIVEA MERFGSRNGK TSKKITIADC GQLE. It is sometimes possible for the material contained within the vial of "Peptidyl-prolyl cis-trans isomerase A, Recombinant Protein" to become dispersed throughout the inside of the vial, particularly around the seal of said vial, during shipment and storage. We always suggest centrifuging these vials to consolidate all of the liquid away from the lid and to the bottom of the vial prior to opening. Please be advised that certain products may require dry ice for shipping and that, if this is the case, an additional dry ice fee may also be required.Precautions
All products in the AAA Biotech catalog are strictly for research-use only, and are absolutely not suitable for use in any sort of medical, therapeutic, prophylactic, in-vivo, or diagnostic capacity. By purchasing a product from AAA Biotech, you are explicitly certifying that said products will be properly tested and used in line with industry standard. AAA Biotech and its authorized distribution partners reserve the right to refuse to fulfill any order if we have any indication that a purchaser may be intending to use a product outside of our accepted criteria.Disclaimer
Though we do strive to guarantee the information represented in this datasheet, AAA Biotech cannot be held responsible for any oversights or imprecisions. AAA Biotech reserves the right to adjust any aspect of this datasheet at any time and without notice. It is the responsibility of the customer to inform AAA Biotech of any product performance issues observed or experienced within 30 days of receipt of said product. To see additional details on this or any of our other policies, please see our Terms & Conditions page.Item has been added to Shopping Cart
If you are ready to order, navigate to Shopping Cart and get ready to checkout.