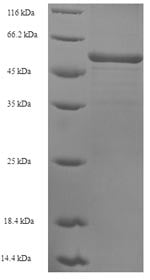
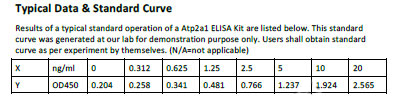
Sarcoplasmic/endoplasmic reticulum calcium ATPase 2 Recombinant Protein | ATP2A2 recombinant protein
Recombinant Human Sarcoplasmic/endoplasmic reticulum calcium ATPase 2
Gene Names
ATP2A2; DD; DAR; ATP2B; SERCA2
Purity
Greater or equal to 85% purity as determined by SDS-PAGE.
Synonyms
Sarcoplasmic/endoplasmic reticulum calcium ATPase 2; N/A; Recombinant Human Sarcoplasmic/endoplasmic reticulum calcium ATPase 2; Calcium pump 2; Calcium-transporting ATPase sarcoplasmic reticulum type, slow twitch skeletal muscle isoform; Endoplasmic reticulum class 1/2 Ca(2+) ATPase; ATP2A2 recombinant protein
Host
E Coli or Yeast or Baculovirus or Mammalian Cell
Purity/Purification
Greater or equal to 85% purity as determined by SDS-PAGE.
Form/Format
Lyophilized or liquid (Format to be determined during the manufacturing process)
Sequence Positions
314-756aa; Partial
Sequence
VITTCLALGTRRMAKKNAIVRSLPSVETLGCTSVICSDKTGTLTTNQMSVCRMFILDRVEGDTCSLNEFTITGSTYAPIGEVHKDDKPVNCHQYDGLVELATICALCNDSALDYNEAKGVYEKVGEATETALTCLVEKMNVFDTELKGLSKIERANACNSVIKQLMKKEFTLEFSRDRKSMSVYCTPNKPSRTSMSKMFVKGAPEGVIDRCTHIRVGSTKVPMTSGVKQKIMSVIREWGSGSDTLRCLALATHDNPLRREEMHLEDSANFIKYETNLTFVGCVGMLDPPRIEVASSVKLCRQAGIRVIMITGDNKGTAVAICRRIGIFGQDEDVTSKAFTGREFDELNPSAQRDACLNARCFARVEPSHKSKIVEFLQSFDEITAMTGDGVNDAPALKKAEIGIAMGSGTAVAKTASEMVLADDNFSTIVAAVEEGRAIYNNM
Preparation and Storage
Store at -20 degree C, for extended storage, conserve at -20 degree C or -80 degree C.
Related Product Information for ATP2A2 recombinant protein
This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the translocation of calcium from the cytosol to the sarcoplasmic reticulum lumen. Isoform 2 is involved in the regulation of the contraction/relaxation cycle.
Product Categories/Family for ATP2A2 recombinant protein
References
Molecular cloning of cDNAs from human kidney coding for two alternatively spliced products of the cardiac Ca2+-ATPase gene.Lytton J., Maclennan D.H.J. Biol. Chem. 263:15024-15031(1988) The finished DNA sequence of human chromosome 12.Scherer S.E., Muzny D.M., Buhay C.J., Chen R., Cree A., Ding Y., Dugan-Rocha S., Gill R., Gunaratne P., Harris R.A., Hawes A.C., Hernandez J., Hodgson A.V., Hume J., Jackson A., Khan Z.M., Kovar-Smith C., Lewis L.R., Lozado R.J., Metzker M.L., Milosavljevic A., Miner G.R., Montgomery K.T., Morgan M.B., Nazareth L.V., Scott G., Sodergren E., Song X.-Z., Steffen D., Lovering R.C., Wheeler D.A., Worley K.C., Yuan Y., Zhang Z., Adams C.Q., Ansari-Lari M.A., Ayele M., Brown M.J., Chen G., Chen Z., Clerc-Blankenburg K.P., Davis C., Delgado O., Dinh H.H., Draper H., Gonzalez-Garay M.L., Havlak P., Jackson L.R., Jacob L.S., Kelly S.H., Li L., Li Z., Liu J., Liu W., Lu J., Maheshwari M., Nguyen B.-V., Okwuonu G.O., Pasternak S., Perez L.M., Plopper F.J.H., Santibanez J., Shen H., Tabor P.E., Verduzco D., Waldron L., Wang Q., Williams G.A., Zhang J., Zhou J., Allen C.C., Amin A.G., Anyalebechi V., Bailey M., Barbaria J.A., Bimage K.E., Bryant N.P., Burch P.E., Burkett C.E., Burrell K.L., Calderon E., Cardenas V., Carter K., Casias K., Cavazos I., Cavazos S.R., Ceasar H., Chacko J., Chan S.N., Chavez D., Christopoulos C., Chu J., Cockrell R., Cox C.D., Dang M., Dathorne S.R., David R., Davis C.M., Davy-Carroll L., Deshazo D.R., Donlin J.E., D'Souza L., Eaves K.A., Egan A., Emery-Cohen A.J., Escotto M., Flagg N., Forbes L.D., Gabisi A.M., Garza M., Hamilton C., Henderson N., Hernandez O., Hines S., Hogues M.E., Huang M., Idlebird D.G., Johnson R., Jolivet A., Jones S., Kagan R., King L.M., Leal B., Lebow H., Lee S., LeVan J.M., Lewis L.C., London P., Lorensuhewa L.M., Loulseged H., Lovett D.A., Lucier A., Lucier R.L., Ma J., Madu R.C., Mapua P., Martindale A.D., Martinez E., Massey E., Mawhiney S., Meador M.G., Mendez S., Mercado C., Mercado I.C., Merritt C.E., Miner Z.L., Minja E., Mitchell T., Mohabbat F., Mohabbat K., Montgomery B., Moore N., Morris S., Munidasa M., Ngo R.N., Nguyen N.B., Nickerson E., Nwaokelemeh O.O., Nwokenkwo S., Obregon M., Oguh M., Oragunye N., Oviedo R.J., Parish B.J., Parker D.N., Parrish J., Parks K.L., Paul H.A., Payton B.A., Perez A., Perrin W., Pickens A., Primus E.L., Pu L.-L., Puazo M., Quiles M.M., Quiroz J.B., Rabata D., Reeves K., Ruiz S.J., Shao H., Sisson I., Sonaike T., Sorelle R.P., Sutton A.E., Svatek A.F., Svetz L.A., Tamerisa K.S., Taylor T.R., Teague B., Thomas N., Thorn R.D., Trejos Z.Y., Trevino B.K., Ukegbu O.N., Urban J.B., Vasquez L.I., Vera V.A., Villasana D.M., Wang L., Ward-Moore S., Warren J.T., Wei X., White F., Williamson A.L., Wleczyk R., Wooden H.S., Wooden S.H., Yen J., Yoon L., Yoon V., Zorrilla S.E., Nelson D., Kucherlapati R., Weinstock G., Gibbs R.A.Nature 440:346-351(2006) Complete sequencing and characterization of 21,243 full-length human cDNAs.Ota T., Suzuki Y., Nishikawa T., Otsuki T., Sugiyama T., Irie R., Wakamatsu A., Hayashi K., Sato H., Nagai K., Kimura K., Makita H., Sekine M., Obayashi M., Nishi T., Shibahara T., Tanaka T., Ishii S., Yamamoto J., Saito K., Kawai Y., Isono Y., Nakamura Y., Nagahari K., Murakami K., Yasuda T., Iwayanagi T., Wagatsuma M., Shiratori A., Sudo H., Hosoiri T., Kaku Y., Kodaira H., Kondo H., Sugawara M., Takahashi M., Kanda K., Yokoi T., Furuya T., Kikkawa E., Omura Y., Abe K., Kamihara K., Katsuta N., Sato K., Tanikawa M., Yamazaki M., Ninomiya K., Ishibashi T., Yamashita H., Murakawa K., Fujimori K., Tanai H., Kimata M., Watanabe M., Hiraoka S., Chiba Y., Ishida S., Ono Y., Takiguchi S., Watanabe S., Yosida M., Hotuta T., Kusano J., Kanehori K., Takahashi-Fujii A., Hara H., Tanase T.-O., Nomura Y., Togiya S., Komai F., Hara R., Takeuchi K., Arita M., Imose N., Musashino K., Yuuki H., Oshima A., Sasaki N., Aotsuka S., Yoshikawa Y., Matsunawa H., Ichihara T., Shiohata N., Sano S., Moriya S., Momiyama H., Satoh N., Takami S., Terashima Y., Suzuki O., Nakagawa S., Senoh A., Mizoguchi H., Goto Y., Shimizu F., Wakebe H., Hishigaki H., Watanabe T., Sugiyama A., Takemoto M., Kawakami B., Yamazaki M., Watanabe K., Kumagai A., Itakura S., Fukuzumi Y., Fujimori Y., Komiyama M., Tashiro H., Tanigami A., Fujiwara T., Ono T., Yamada K., Fujii Y., Ozaki K., Hirao M., Ohmori Y., Kawabata A., Hikiji T., Kobatake N., Inagaki H., Ikema Y., Okamoto S., Okitani R., Kawakami T., Noguchi S., Itoh T., Shigeta K., Senba T., Matsumura K., Nakajima Y., Mizuno T., Morinaga M., Sasaki M., Togashi T., Oyama M., Hata H., Watanabe M., Komatsu T., Mizushima-Sugano J., Satoh T., Shirai Y., Takahashi Y., Nakagawa K., Okumura K., Nagase T., Nomura N., Kikuchi H., Masuho Y., Yamashita R., Nakai K., Yada T., Nakamura Y., Ohara O., Isogai T., Sugano S.Nat. Genet. 36:40-45(2004) Identification of a new SERCA2 splice variant regulated during monocytic differentiation.Gelebart P., Martin V., Enouf J., Papp B.Biochem. Biophys. Res. Commun. 303:676-684(2003) TRAM2 protein interacts with endoplasmic reticulum Ca2+ pump Serca2b and is necessary for collagen type I synthesis.Stefanovic B., Stefanovic L., Schnabl B., Bataller R., Brenner D.A.Mol. Cell. Biol. 24:1758-1768(2004) Detection of sequence-specific tyrosine nitration of manganese SOD and SERCA in cardiovascular disease and aging.Xu S., Ying J., Jiang B., Guo W., Adachi T., Sharov V., Lazar H., Menzoian J., Knyushko T.V., Bigelow D., Schoeneich C., Cohen R.A.Am. J. Physiol. 290:H2220-H2227(2006) Global, in vivo, and site-specific phosphorylation dynamics in signaling networks.Olsen J.V., Blagoev B., Gnad F., Macek B., Kumar C., Mortensen P., Mann M.Cell 127:635-648(2006) Kinase-selective enrichment enables quantitative phosphoproteomics of the kinome across the cell cycle.Daub H., Olsen J.V., Bairlein M., Gnad F., Oppermann F.S., Korner R., Greff Z., Keri G., Stemmann O., Mann M.Mol. Cell 31:438-448(2008) A quantitative atlas of mitotic phosphorylation.Dephoure N., Zhou C., Villen J., Beausoleil S.A., Bakalarski C.E., Elledge S.J., Gygi S.P.Proc. Natl. Acad. Sci. U.S.A. 105:10762-10767(2008) The anti-apoptotic protein HAX-1 interacts with SERCA2 and regulates its protein levels to promote cell survival.Vafiadaki E., Arvanitis D.A., Pagakis S.N., Papalouka V., Sanoudou D., Kontrogianni-Konstantopoulos A., Kranias E.G.Mol. Biol. Cell 20:306-318(2009) Quantitative phosphoproteomic analysis of T cell receptor signaling reveals system-wide modulation of protein-protein interactions.Mayya V., Lundgren D.H., Hwang S.-I., Rezaul K., Wu L., Eng J.K., Rodionov V., Han D.K.Sci. Signal. 2:RA46-RA46(2009) Initial characterization of the human central proteome.Burkard T.R., Planyavsky M., Kaupe I., Breitwieser F.P., Buerckstuemmer T., Bennett K.L., Superti-Furga G., Colinge J.BMC Syst. Biol. 5:17-17(2011) POST, partner of stromal interaction molecule 1 (STIM1) , targets STIM1 to multiple transporters.Krapivinsky G., Krapivinsky L., Stotz S.C., Manasian Y., Clapham D.E.Proc. Natl. Acad. Sci. U.S.A. 108:19234-19239(2011) An enzyme assisted RP-RPLC approach for in-depth analysis of human liver phosphoproteome.Bian Y., Song C., Cheng K., Dong M., Wang F., Huang J., Sun D., Wang L., Ye M., Zou H.J. Proteomics 96:253-262(2014) Spectrum of novel ATP2A2 mutations in patients with Darier's disease.Sakuntabhai A., Burge S., Monk S., Hovnanian A.Hum. Mol. Genet. 8:1611-1619(1999) ATP2A2 mutations in Darier's disease variant cutaneous phenotypes are associated with missense mutations, but neuropsychiatric features are independent of mutation class.Ruiz-Perez V.L., Carter S.A., Healy E., Todd C., Rees J.L., Steijlen P.M., Carmichael A.J., Lewis H.M., Hohl D., Itin P., Vahlquist A., Gobello T., Mazzanti C., Reggazini R., Nagy G., Munro C.S., Strachan T.Hum. Mol. Genet. 8:1621-1630(1999) ATP2A2 mutations in Darier's disease and their relationship to neuropsychiatric phenotypes.Jacobsen N.J.O., Lyons I., Hoogendoorn B., Burge S., Kwok P.-Y., O'Donovan M.C., Craddock N., Owen M.J.Hum. Mol. Genet. 8:1631-1636(1999) Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease.Sakuntabhai A., Ruiz-Perez V., Carter S., Jacobsen N., Burge S., Monk S., Smith M., Munro C.S., O'Donovan M.C., Craddock N., Kucherlapati R., Rees J.L., Owen M.J., Lathrop G.M., Monaco A.P., Strachan T., Hovnanian A.Nat. Genet. 21:271-277(1999) Acrokeratosis verruciformis of Hopf is caused by mutation in ATP2A2 evidence that it is allelic to Darier's disease.Dhitavat J., Macfarlane S., Dode L., Leslie N., Sakuntabhai A., MacSween R., Saihan E., Hovnanian A.J. Invest. Dermatol. 120:229-232(2003) Ca2+-ATPases in non-failing and failing heart evidence for a novel cardiac sarco/endoplasmic reticulum Ca2+-ATPase 2 isoform (SERCA2c) .Dally S., Bredoux R., Corvazier E., Andersen J.P., Clausen J.D., Dode L., Fanchaouy M., Gelebart P., Monceau V., Del Monte F., Gwathmey J.K., Hajjar R., Chaabane C., Bobe R., Raies A., Enouf J.Biochem. J. 395:249-258(2006) Three-base deletion mutation c.120_122delGTT in ATP2A2 leads to the unique phenotype of comedonal Darier disease.Tsuruta D., Akiyama M., Ishida-Yamamoto A., Imanishi H., Mizuno N., Sowa J., Kobayashi H., Ishii M., Kurokawa I., Shimizu H.Br. J. Dermatol. 162:687-689(2010) +Additional computationally mapped references.<p>Provides general information on the entry.
NCBI and Uniprot Product Information
NCBI GI #
NCBI GeneID
NCBI Accession #
NCBI GenBank Nucleotide #
Molecular Weight
52.3 kDa
NCBI Official Full Name
sarcoplasmic/endoplasmic reticulum calcium ATPase 2 isoform a
NCBI Official Synonym Full Names
ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2
NCBI Official Symbol
ATP2A2
NCBI Official Synonym Symbols
DD; DAR; ATP2B; SERCA2
NCBI Protein Information
sarcoplasmic/endoplasmic reticulum calcium ATPase 2
UniProt Protein Name
Sarcoplasmic/endoplasmic reticulum calcium ATPase 2
UniProt Gene Name
ATP2A2
UniProt Synonym Gene Names
ATP2B; SERCA2; SR Ca(2+)-ATPase 2
UniProt Entry Name
AT2A2_HUMAN
Similar Products
Product Notes
The ATP2A2 atp2a2 (Catalog #AAA113752) is a Recombinant Protein produced from E Coli or Yeast or Baculovirus or Mammalian Cell and is intended for research purposes only. The product is available for immediate purchase. The immunogen sequence is 314-756aa; Partial. The amino acid sequence is listed below: VITTCLALGT RRMAKKNAIV RSLPSVETLG CTSVICSDKT GTLTTNQMSV CRMFILDRVE GDTCSLNEFT ITGSTYAPIG EVHKDDKPVN CHQYDGLVEL ATICALCNDS ALDYNEAKGV YEKVGEATET ALTCLVEKMN VFDTELKGLS KIERANACNS VIKQLMKKEF TLEFSRDRKS MSVYCTPNKP SRTSMSKMFV KGAPEGVIDR CTHIRVGSTK VPMTSGVKQK IMSVIREWGS GSDTLRCLAL ATHDNPLRRE EMHLEDSANF IKYETNLTFV GCVGMLDPPR IEVASSVKLC RQAGIRVIMI TGDNKGTAVA ICRRIGIFGQ DEDVTSKAFT GREFDELNPS AQRDACLNAR CFARVEPSHK SKIVEFLQSF DEITAMTGDG VNDAPALKKA EIGIAMGSGT AVAKTASEMV LADDNFSTIV AAVEEGRAIY NNM. It is sometimes possible for the material contained within the vial of "Sarcoplasmic/endoplasmic reticulum calcium ATPase 2, Recombinant Protein" to become dispersed throughout the inside of the vial, particularly around the seal of said vial, during shipment and storage. We always suggest centrifuging these vials to consolidate all of the liquid away from the lid and to the bottom of the vial prior to opening. Please be advised that certain products may require dry ice for shipping and that, if this is the case, an additional dry ice fee may also be required.Precautions
All products in the AAA Biotech catalog are strictly for research-use only, and are absolutely not suitable for use in any sort of medical, therapeutic, prophylactic, in-vivo, or diagnostic capacity. By purchasing a product from AAA Biotech, you are explicitly certifying that said products will be properly tested and used in line with industry standard. AAA Biotech and its authorized distribution partners reserve the right to refuse to fulfill any order if we have any indication that a purchaser may be intending to use a product outside of our accepted criteria.Disclaimer
Though we do strive to guarantee the information represented in this datasheet, AAA Biotech cannot be held responsible for any oversights or imprecisions. AAA Biotech reserves the right to adjust any aspect of this datasheet at any time and without notice. It is the responsibility of the customer to inform AAA Biotech of any product performance issues observed or experienced within 30 days of receipt of said product. To see additional details on this or any of our other policies, please see our Terms & Conditions page.Item has been added to Shopping Cart
If you are ready to order, navigate to Shopping Cart and get ready to checkout.